 Blue and dark blue clay and vivianite is an instrument of litotherapy
Blue and dark blue clay and vivianite is an instrument of litotherapy
Blue and dark blue clay and vivianite (there is radium) -->rus
 Vivianite (greyish water phosphate of iron Fe3 (PO4) 2 * 8 H2O) got naming after J.G. Vivian the English mineralogist which first found out this stone. A vivianite is a dark blue bog iron-stone, dark blue earth. A vivianite has other names - glaukosiderite, myullicit. A mineral has bluish-green, dark blue tints (impurity). There are colourless stone, recently obtained from under earth. Under sunbeams a vivianite darkles notedly, while will not become black practically (under the action of radiation violet fluorite darkles is ftorid of calcium of CaF2 and quartz an amethyst is an oxide of silicium of SiO2, becoming violet-black practically). A mineral has glass, mother-of-pearl brilliance. At storage in metallic tableware and opened contact along with iron Fe active oxidization of iron goes due to water, contained in a vivianite, and loss of water a vivianite. A vivianite is water-contain salt (phosphates contain water of H2O and alkaline grounds of OH often).
Vivianite (greyish water phosphate of iron Fe3 (PO4) 2 * 8 H2O) got naming after J.G. Vivian the English mineralogist which first found out this stone. A vivianite is a dark blue bog iron-stone, dark blue earth. A vivianite has other names - glaukosiderite, myullicit. A mineral has bluish-green, dark blue tints (impurity). There are colourless stone, recently obtained from under earth. Under sunbeams a vivianite darkles notedly, while will not become black practically (under the action of radiation violet fluorite darkles is ftorid of calcium of CaF2 and quartz an amethyst is an oxide of silicium of SiO2, becoming violet-black practically). A mineral has glass, mother-of-pearl brilliance. At storage in metallic tableware and opened contact along with iron Fe active oxidization of iron goes due to water, contained in a vivianite, and loss of water a vivianite. A vivianite is water-contain salt (phosphates contain water of H2O and alkaline grounds of OH often).
Like a vivianite the mineral of kakoksen Fe4 (PO4) 3 (OH) 3 looks chemical composition * 12 H2O - also phosphate of iron, enhydrous and alkaline foundation. Kakoksen is presented by needle-shaped crystals, fibred crusts and radiant aggregates of brown-yellow, goldish-zheltogo, rozovato-zheltogo color. Meets as the second mineral, present in pegmatites, containing phosphates, in an association with dyufrenite, rockbridzhite, wavellite, shtrengite, sometimes kakoksen is associated with swamp ore (water oxides of iron). He forms the yellow or light brown-yellow brushes of flattened crystals of monoclinic system. Beautiful rare mineral, reminds plants. A phosphate also is famous turquoise - Cu Al6 (PO4) 4 (OH) 8 * 4 H2O, water phosphate of copper and aluminium with alkaline foundation, which becomes brighter from water, to the isomorphous row of which a chalcosiderite is near - Cu Fe6 (PO4) 4 (OH) 8 * 4 H2O (look like a vivianite).
Phosphates. In this category, besides phosphates (minerals, containing the phosphoric oxygen group of PO4), belong also arsenaty and vanadaty (minerals, containing the groups of arsenic AsO4 and vanadium of VO4 with oxygen). Between these connections there are transitional isomorphous forms and rows - from phosphates to arsenatam (arsenic), from arsenatov to vanadatam and, rarer, from vanadatov (vanadium V) to the phosphates (P). In the earth's crust primary phosphorus is present mainly in composition an apatite which is widespread almost in all of orthorocks, in pegmatitic tendons at kal'der of volcanos and in some ore deposits. The processes of weathering, dissolution and transfer provide the accumulation of phosphates in soils and salt water. Living organisms extract from them phosphorus which is needed for their existence. Accumulations of remains of organisms and they excrements are the bed of commercial-size. Arsenaty and vanadaty - mainly the second minerals, appearing in the deposits of complex composition, enriched dry sulfides, mainly arsenic and cobalt. Admixtures in blue and siney to clay.
A vivianite appears in places, near to the terrene - under earth, under influence of oxygen of air (partial aeraciya). His variety black kertschenite is formed practically outdoors in the sunshine (Ukraine, CIS). Circulatory at a surface earths rich in phosphates underwaters affect minerals which contain iron (mainly, bivalent) is a brazil, siderit, pirrotin and wash part of iron. When water evaporates almost fully, mineral a vivianite is crystallized. In pegmatites (granit intrusion) the phosphates of iron can grow into a vivianite, absorbing, taking in and partly linking water. In a fresh kind a vivianite is colourless, but due to the second changes begins quickly to acquire the blue, bluish-green and light-blue colouring. Many crystals are transparent.
A vivianite can be formed as the second metamorphic and sedimentary mineral is in the top of many sulfide (dry) deposits mines, and also at the second changes of orthomonophosphates of pegmatites (metamorfity on hot batholite, volcanos, magmatic kimberlitakh with calderas of water). He appears, in addition, in the clay deposits of fresh lakes as a result of interaction of the water enriched iron with phosphoprous-containing organic material at banks (cane-brakes and other) and at Priodaxum and near pond of the fields, before abundantly fertilized phosphorus. A vivianite is also set in the beds of lignitov and sedimentary rocks iron-stones of freshwater pools on land.
Meeting a mineral is possible as needle-shaped and lamellar crystals usually green color (impurity). However on air because of oxidization a mineral is changed by a color from green to indigo-dark blue. In presence air dark blue-green aggregates are formed. Meeting is possible as fibred and radiant aggregates, earthy marbles and tumours, concretions. The basic deposit mines of this mineral is consider Ukraine, Russia, USA, Britain.
Application. A vivianite is used as collection material, mineral pigment for making of greyish-dark blue paint (natural grey-dark blue an indigo). Dark blue-black vivianity name kertschenite (Ukraine, CIS). In the iron-stones of vianite and kertschenite (water phosphate of iron) is a harmful admixture from maintenance of phosphorus and active water (temperature high and martin furnaces, powder-like technologies, vacuum and ultrahigh temperatures). A vivianite is water-contain phosphoric salt the type of which is actively studied from the end of XIX century - possible deposits on land of ocean, marine and water salts.
It is partly used from a website http://yakginka.ru
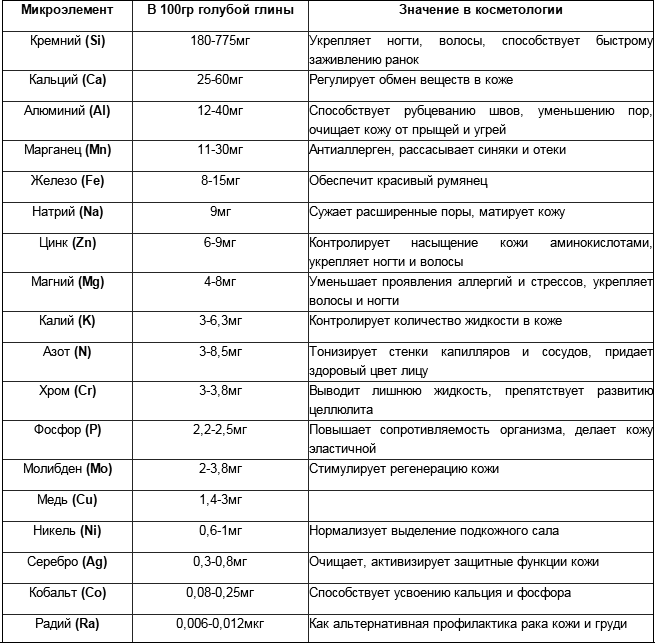
Blue and dark blue clay (with a vivianite is a water phosphate of iron of Fe3 (PO4) 2 * 8 H2) is applied both outwardly - maximally safely and sometimes inward (strictly on purpose of doctor). Blue clay in the composition has plenty of impurity - oligoelementss and mineral matters. What darker dark blue clay, the anymore in it maintenance of radium of Ra (he is dangerous in high doses). What lighter clay, the a more kaolin is in it (clay is in a clean kind). That, than more high-order clay, the it darker and more nasyschena by different minerals. Badly consonant with an alcohol and poisons (as some de bene esse-edible mushrooms of type Coprinus horsetail, dung beetles, dumble beetle, dorbeetle, shaggy cap). Table of maintenance of oligoelementss in siney taken clay from a site http://cosmetic.ua/golubaja_glina_v_kosmetike
Keeping blue and dark blue clay (actually with a vivianite) is needed in bottletight tableware (a vivianite contains Unite in crystals water). Blue clay has contra-indications. It is needed to limit application of blue clay at sharp inflammatory processes, at the diseases of heart, at violations of work of buds, at hypertensive illnesses (provyshennoe pressure hypertension), at the diseases of thyroid (endocrine disorders). It is yet desirable to mark that an erubescence goes at the outward use. Preparing clay for outward application and coating of areas of body is possible from dry clay and water. It is needed to add clean water to consistency of sour cream, mixing a wooden stick. Essential oils and other mineral impurity do not add. In the sunshine blue and dark blue clays darkle (vivianite).
Smearing clay on a body is necessary a thick layer, that it did not dry out -dlya it is even better to do it compress from a cellophane, to turn the area of body, clayed, celofanom. Dissolving blue clay is necessary clean fresh water and desirably in clay or glass tableware (if there is not such, in enameled or plastic). Blue clay for hairs. To inflict the same clay a thick mask on hairs (derma of hairs). To cover a plastic package, that clay did not dry (absence of effect of mankurta) out, on 30-45 minutes, after to wash off usual water.
It is partly used from a website http://domovouyasha.ru/golubaya-glina-lechebnyie-svoystva-I-primenenie
Radium Ra is dangerous in high doses and is chemically an active radio-active element, blighting in high doses. As an active element, can be used for treatment of some forms of shrine (chemotherapy). Plutonium is the almost most radiotoxic element from all of actinoids, however considered the much most not dangerous radio-active element, so radium almost the most poisonous isotope of plutonium is more dangerous in thousand one times - 239 Pu. His connections are radiation-poisonous, that is investigation α-radiation. Alpha-particles present a danger in case that their source is in a body or close to the body of man, they damage circumferential the dangerous element of biological tissue and DNA (deoxyribonucleic acid) of organism. A gamma-radiation is less dangerously for an organism. Radium Ra sometimes is the product of isotopic disintegration of radio-active uranium and meets on the deposits of uranium and nasturane pitchblende. Uraninit (oxide of uranium of UO2) often contains radium and is with a greenish and bluish tint.
Radium is an element of main sub-group of the second group of seventh period of the periodic system of chemical elements of D.I. Mendeleyev, with an atomic number 88. Designated character of Ra (lat. Radium). Radium is a CAS-number: 7440-14-4 is a brilliant alkaline-earth radio-active metal of serebristo-belogo color, dimming on air (oxidizes). Possesses high chemical activity (he is used in khimeotherapy). Radio-active, his isotope-nuclide of 226ra is most steady is a half-period 1600 years. From the group of plutonium. Geochemistry of radium is in a great deal determined the features of migration and concentration of primary uranium and chemical properties of radium - chemically-active alkaline-earth radiogenic radio-active metal.
Radium-223 actinium-Х (AcX) series uranium-235 11,435 day alpha radon-219 (actinon, An)
Radium-224 thorium-Х (ThX) series thorium-232 3,66 day alpha radon-220 (thoron, Tn)
Radium-226 Radium (Ra) series uranium-238 1602 years alpha radon-222 (radon, Rn)
Radium-228 mesothorium-I (MsTh1) series thorium-232 5,75 years beta actinium-228 (mesothorium II, MsTh2)
Among processes, cooperant the concentration of radium, it is necessary to specify on forming on the small depths of geochemical barriers radium is concentrated in which. Such barriers can be, for example, sulfate barriers in the area of oxidization and area of forming of clays. Risings from below khloridnye sulphuretted hydrogen radiysoderzhaschie waters in the area of oxidization become a sulfate, radium is besieged with BaSO4 and CaSO4, where he becomes the insoluble permanent source of radon practically. From the high migratory capacity of uranium and ability of him for a concentration many types of uranium formation of ore are formed in hydrothermal, coals, bitumens, crigglings, sandstones, peatbogs, phosphorite, brown ironstone (bog iron ore, brown haematite), clays with bone tailings of finfishess (lithofacies). At incineration of coals an ash and slags is enriched the isotope of radium of 226ra, similarly as well as at rotting of vegetable tailings.
After 1 g of radium more than 200 kg of gold paid at the beginning of XX century. Usually radium in passing is obtained from uranium ores. In old enough ores there are 333 milligrams of radium-226 on the ton of uranium. In 1954 the world supply of all of the obtained radium was estimated in 2,5 kg Radium at normal terms is a brilliant white metal, on air dims (probably, because of formation of nitrida of radium). Reacts with water. Behaves like a barium (popular in the USA) and strontium (strontium is contained in celestine - sulfate of strontium), but more chemically active. Ordinary degree of oxidization +2. Gidroksid of radium of Ra(OH) 2 is strong corrosive foundation. Presently radium is used in the compact sources of neutrons, for this purpose his two-bits are alloyed with a beryllium.
In medicine radium is used as a source of radon for preparation of radon baths (frequent sputnik of khimeotherapy). Radium is applied for a brief irradiation at treatment of malignant diseases of derma, mucous membrane of nose, mochepolovogo highway. Analogues radionuklidy with necessary properties which get on accelerating and in nuclear reactor- 60 CO (T1/2 = 5,3), 137 Cs (T1/2 = 30,2), 182 Ta (T1/2 = 115 sut.), 192 Ir (T1/2 = 74 sut.), 198 Au (T1/2 = 2,7 sut.) et cetera To 70th of XX century radium was used for making of luminous paints of permanent luminescence (for razmetki of clock-faces of aviation and marine devices, special clock and other), now he is sometimes replaced tritium of group of lithium (T1/2 = 12,3) and 147 Pm (T1/2 = 2,6) is promethium. Radium svetomassu it is possible to meet in old fir-tree toys, tumblerakh with radiation from beneath of tag of lever, on the scales of old radio receivers. From high radio-activity radium and his connections spontaneously shine in darkness (fosforiciruyut). A cost of radium from data of XX century is 12 million dollars for a gramme. Dearest metal in the world is a californium-252, his price varies from 6,5 to 27 million dollars for a gramme (we will confront at price with radium).
Radium Ra is extraordinarily radiotoxicity. In an organism he behaves like a calcium - an about 80% entering organism radium Ra accumulates in bone stock. The large concentrations of radium cause an osteoporosis (also is investigation of treatment of shrine radium), the breaks of bones and malignant tumours of bones and hematopoietic system are present. A danger presents a radon - gaseous radio-active product of disintegration of radium Ra also (present on the deposits of uranium and radium).
At development of uranium deposit stratal and begun to the swing waters intensively enter oil layers, interphase water-oil is increased, and as a result radium goes away to the stream of filter-passing waters. At enhanceable maintenance of sulphate-ionov cut-in in water radium and barium is besieged as radiobarita of Ba(Ra) SO4, which falls out on-the-spot pipes, armature, reservoirs and look like usual scum - very dear at price. Typical by volume activity of acting on a surface vodoneftyanoy mixture on 226Ra and 228Ra can be 10 Bk/l (corresponds liquid radio-active offcuts). The table of contents of radium is enhanceable in phosphatic breeds (vivianity), mineral the celsian of BaAl2Si2O8 is very rare, the accumulation of radium with formation of stable radium feldspars (granites and pegmatites) and minerals does not take a place from a short half-period radium. A bulk of radium is in the dissipated consisting of mountain breeds.
It is partly used from a website https://ru.wikipedia.org/wiki/%D0%A0%D0%B0%D0%B4%D0%B8%D0%B9
Those, for whom radium is and who he needs are scientists and doctors - will not part with the grains of radium salts and radium not for all the the money in the world. In Katang (Congo, Africa) there is plenty enough of radium in the enormous beds of uranium ore. From 500 tons of primary ore a 1 gramme of radioaktive radium Ra turns out as his salts in mixture with a barium and chlorine and for this purpose it is needed to use up 500 tons of chemical reagents, 10 000 tons of the distilled water, skilled labour is 150 persons during a month and enormous amount of electric power. To extract clean radium, work of large group of specialists-chemists is needed during 5 months, and then - 4 months for preparation of him to the actual use. Indeed "in a gramme booty - in a year labours". a 1 gramme of radium is a piece of metal measuring with the tag of fountain-pen.
It is necessary to consider Anri Dominichi the father of radio-therapy. In 1906 he discovered that the rays of radium, filtered through two leaden plates (to cut off alpha- and beta ray, and also gamma-rays of small energy), can be used for destruction of tumour biological tissues. You will ask doctors about radium, and they will say you, that it is one of surprisings facilities for treatment of various diseases, from some time gettings in hands physicians. Because there is very little radium, to use him constantly for the irradiation of all of tumours beside the purpose. Instead on not dangerous tumours a radon - rare radio-active gas, appearing at disintegration of radium is used.
For 1 one gramme of radium Ra is made by about 1 160 000 calories of heat is an enough body, to take 10 litres of icy water to boiling (he is heated - radio-active). Thus radium will lose the about 1/2500 weight on the expended thermal energy. Radiating, radium changes chemically. From very rare and very dear metal he gradually grows into stable lead of Pb, which at absorption of elementary particles and radiation again slowly grows into radium and less heavy radio-active metals (lead comes forward including as absorbers of elementary particles in the nuclear reactors of modern AES and is at the sources of nuclear and thermonuclear explosions). Ordinary "transmutation of radium Ra in one side" occupies about 3460 years is a span of time between XXI century and Ancient Egypt.
It is partly used from a website http://etolen.com/
Blue clay is more frequent than all used in medicine. There is plenty of salts of cadmium and cobalt in this type of clay. Blue clay found a wideuse in a cosmeticology. Blue clay is used for different masks for clearing of derma and from an ugrevoy rash. Masks with blue clay strengthen and pull up a derma, even out wrinkles, take off an irritation, contest with cellyulitom and superfluous weight. Application of blue clay is many-sided. Clay is used not only in a cosmeticology but also for treatment of illnesses of joints, muscles, spine, remaining phenomena of poliomyelitis, at traumas, gynaecological diseases, locks, inflammation of gall-bladder.
Folk medicine usees blue clay in treatment of leucosises, tumours, adenoids, inflammation of lymphonoduss (cancer and malignant diseases are radium and his isotopes), it is treat anaemia, atherosclerosis, head pain, paralysis, epilepsy, nervous break-downs, inflammation of ear, illnesses of brain, diabetes, illnesses, cold, bleeding, quinsies, different derma diseases, illnesses of joints, ulcers and other great deal. At the heel spurs of leg it is needed to steam out in hot water, whereupon to coat a foot clay ointment or impose clay tiles on the area of spurs. Compresses from blue clay use for treatment of radikulita, rheumatism, gout, arthritis. Blue clay is eaten (as sorbent and malignant diseases) sometimes - by small doses, clay can contain radio-active components (radiation chemotherapy). Additionally buy oil of cypress and dill and treat oneself a celandine.
It is partly used from a website http://milovar-spb.ru/gliny-kaoliny-talki.html

Oboznovskiy careers is Kirovogradskoe mine-administration, clays and its booty by an industrial method. Blue clay is applied both outwardly and inward. The method of application wholly depends on a disease. For treatment of some diseases combine both methods. Outwardly blue clay is applied mainly as washes, coating, rubbing and baths. Clay operates on the sick place of iscelyayusche, cures hyposthenic or sick cages, renews organs healthy and strong cages, hammering into itself dyings off together with dirt and slags. For application a clean loam is inward (very neatly) used without the impurity of sand and similar extraneous including. It is better to use clay, taken one whole piece. To break up a preform on little pieces which by a mortar or bottle to pound in powder. Then to sift through a sieve, to deliver from impurity. To propose the clean prepared powder in the sunshine. In such kind clay is ready to the use. The amount of the applied clay needs to be regulated depending on the necessities of organism.
It is partly used from a website http://kaolincentre.com.ua/kaolin-info/glina-blue-golubaya-primenenie.html
Modern application siney and blue clay and vivianite in medicine. Medicine. E.P. Frimend applied peat in mixture with a bog and lacustrine, and also by seashore clay at the diseases of sciatic nerve, osteomielite and osteoarticular illnesses.
A bit about clay. Lito-period is time of mastering plants and animals of sial shell of dry land. Engulfs all of geological periods of mesozoic era: a trias (180-225 million years back), jurassic (135-180 million years back), swept (70-135 million years back). His duration is about 160 million years. Lito-period was characteristic seas, leavings the powerful deposits of jurassic clay and chalk. On the naked breeds of sial a soil cover, populated microorganisms, is gradually formed. Dryness of climate increased and the process of biotproducts went down simultaneously, and with him and rates of accumulation of vegetable tailings. On this stage there was becoming of modern flora and fauna is an outlet of life on dry land. Basic transformations to the vegetable world were directed on forming of surface rootages, capable actively to master the layer of mineral substratum and provide mastering the plants of dry land.
At the beginning of golocena in a subarctic period, 9800-12000 years back a climate on Earth changed sharply, became more warm. A rise in the temperature of climate was caused by a deglaciation, coverings a powerful ice armour greater part of modern Russia and north of Eurasia. A glacier degraded and retreated long. After itself he left a graded mountain plain, exposed to the erosive washing away by the streams of the melted waters, with the deeply cut in multiterrace river valleys, numerous lakes, exterminated hills and ridges. All is naked, emptily, in place of soil on terrenes - deposits of sands, clays and boulders.
Swamping of lakes. The first and most ancient way is formation of bogs from lakes and ponds, begun after a glacier, proceeds till then. The process of swamping described V. Varlamov in a popular magazine in 1984 g.: the "Recessive glacier left after itself lakes, stone bowls, filled crystal water. And immediately a crystal began turbid. Winds poured in him a dust, spores, seed. The ground deposits went. At first sand and clay, borings marls. Interesnoe was then added more, remains of shallow water inhabitants - plankton." A lake is gradual filled from a bottom and from banks by a silt on which with appearance on him of vegetation the process of peat-forming, peat-humus began with formation of bog. A reed Phragmites, cattails grows near water on the banks of lakes and storage pools, in the mouths of the rivers, on low-laying area and transitional bogs, in places, where water running or there are outlets of underwaters. A reed Phragmites, cattails is instrumental in oxidization of organic matters and takes away elements from water (sodium, sulphur, oil, clay and other).

Bogs of tundra. Ashore the Kara sea it is possible to see seashore bogs, inundable salt water at a pileup run-up stream. Here nature is provide "nyasha" are original traps, getting out from which is impossible. Nyasha is the clay, which is washed out waves, interfuses with an old grass and bushes, caused to the fall at-sea, and mixture of liquid-gel consistency fills the burries of bottom and deep pits. Above the pieces of peat float on waves. Nyasha is hammered together at a coast, in the mouths of the rivers and brooks. Disastrous places for deer and people. Large hillocks in a to 5 mcode high are alternated with zapadinami. Dissection of hillocks rotined on a presence in them kernels from a loam and sand, recovered the heat-insulating layer of peat. Hillocks are alternated on bogs with hollow, filled carex-sphagnum and by sphagnum-cotton grass associations.

In Karelia of Russia (CIS) marsh relief is widespread. At melting of ostancev of ice in his body "craters" appeared with glacial water. Then lakes and ostancy were gradually filled sinking. When ice thawed, former lakes became cone-shaped hills-kamami which the funnel-shaped lowerings were disposed between. Characteristic sign of kama bogs - on them lacustrine deposits - sapropel, clay, lacustrine sands bed under the layer of peat.
Why do not bogs give water? Movement of water in peat depends on his permeability to water, which is determined by volume of the largeness of pores, - retard (evaporation can go from volcanic batholite which are warmed-up from below - therefore bogs often burn from below, gas burns from below and the dried up burn from below peatbogs). Peat as compared to other soils behave to sredne- and semi-permeable, I.e. taken in one group together with loams and silt-covered clay sand. Drainage and use of bogs is caused by the decline of water-table. Distance of influence of osushitel'noy network on sands makes a 2-4 km. In loams and clays the affected zone is estimated a size 180-200 m. For a fight against freezings measures are effective: ground (sandsing or glinovanie) peat; increase of humidity of peat.
Barabinskaya a lowland is characterized many natural features. At first, it is an enormous hollow, therefore a climate is different on the day of hollow, windward and leeward slopes, on Vasyuganskoe and Priobskoe of plateau of Russia (CIS). Here more than a rhythm is somewhere expressed in the gidrotermicheskom mode of summer. Duration of rhythm about 32 years, an ascending cycle is characteristic the improvement of terms of life for most cultural plants, descending - worsening of terms. For example, period 1950-1965 was characteristic the slump of rhythm, that showed up in annual negative water balance with natural drainage of many bogs and shallowing of lakes, steppe formation of meadows.
Secondly, Baraba is characterized low natural drained (small density of river network, weak the cut rivers, small slopes of surface), that is instrumental in stagnation of water and formation of bogs. Curiously, that geomorphologist is selected on its territory seven districts on the terms of drained and at six from them (except for Priobskiy plateau) in the name the words of "poor weak drainage appear and not drained".
Thirdly, the presence of powerful (about 1 km) stream of underwaters is instrumental in swamping and solinization (penetration of salt and ocean waters on dry land, especially after stoppings of tsunami and by seepage of water under earth with its migration into dry land) related to him in sands, clay sand of different century and genesis, laid jurassic clay. The degree of participation of pressure waters in the signup of ground-waters is small (10-20 mm/year), I.e. within the limits of 4 % from the size of fallouts, but enormous harm from them in the accumulation of salts on periphery of lakes and bogs, zasolenii of bogs existing and degraded. Bogs in Barabe appeared 9-11 thousand years back and continue to tread on manes and slopes, busy the forests and plough-lands. Up-river bogs and raw meadows be near in Vasyugane and on Priobskiy of plateau with steppe.
A geological structure and tectonics is rendered by enormous influence on the water mode of territory. So, the large bendings of the earth's crust, built the powerful layer of siltages in which superficial and underground waters flow down from adjoining elevation are most swamped. These waters together with the atmospheric sinking create the surplus moistening. Such the large lowerings it is been Mescherskaya, Barabinskaya, Polesskaya and other lowlands. In the districts of distribution of poor sandy deposits, sour granites up-river bogs, forested a pine-tree, are mainly developed. Low-laying area and transitional bogs prevail on clay deposits. In the conditions of rich water-mineral feed, bogs long remain on the low-laying area stage of formation development. For these districts sedge-hypnum bogs are typical.

L.I. Inisheva, B.S. Maslov. "Enigmatic world of bogs" (fragments about clay)
It is separately desirable to remark a concept "Blue earth" are lagoon-river-delta mineral deposits of amber, E.Y. Kievlenko and N.N. Senkevich, "Geology of deposits of semi-precious stone". It is quartz sands of blue color of amber-contain of "blue earth" of the Seashore deposit of Baltic of the CIS. Power of "blue earth" hesitates from 50 see to 20 m. Placed - with siderite. A glauconite is mica, potassium-contain water aluminum silicate, mineral from the group of gidroslyud (contains water) of subclass of the stratified silicates, clay mineral of variable composition of category of micas (silicates) with high maintenance of two- and trivalence ferric iron, calcium, magnesium, potassium, phosphorus, contains more than twenty oligoelementss among which is a copper, silver, nickel, cobalt, manganese, zinc, molybdenum, arsenic (poisonous), chrome, tin, beryllium (not safe), kamdiy (dangerous), et al. Meets in sedimentary rocks scalls. The admixture of mica of glauconite gives containing him breeds a greenish tint. Quartz-glaukonite sands of blue and dark blue color can be with possible dangerous grey glaukodotom is connection of biotaccessible iron at presence of poisonous grey, arsenic and cobalt. Color of glaukodote from grey to white, in the sprinkle of snow - black, a mineral is reminded by Labrador and labradorit (they are radio-active). Glaukodot is a dangerous poisonous mineral (Co, Fe) As S is dry waterless connection of cobalt and iron with an arsenic and sulphur, in an association with cobaltite and chalcopyrite, which can treat in very small doses. At heating glaukodot publishes a garlicky smell.

