Stone, minerals and semiprecious of the world stone
Carbonate: Calcite -->rus
 Diagnostic cart.
Diagnostic cart.
Cа СO3
Crystal structure trigonal
Hardness on the Mohs scale 3
Specific unit weight mass 2,71
Cleavage perfect absolute
Fracture, break padman
Colors colourless, polycoloured (multicoloured)
Colors in powder triturate white
Glance (glitter, glare) from glass to pearl
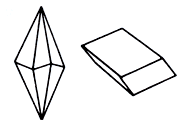
 Calcite - one of the most widespread minerals - presented in a number of crystalline forms. In nature Calcite be found in various forms: his crystals can have a look of prisms, rhombohedrons, scalenohedron, tablets. In addition, different aggregates appear often (grainy, parallel, columnar, basaltiform, lamellar tabular); veins of fibred structure; concretion of zonal addition. Colouring of Calcite is very various - white, reddish, yellow, brown or green.
Calcite - one of the most widespread minerals - presented in a number of crystalline forms. In nature Calcite be found in various forms: his crystals can have a look of prisms, rhombohedrons, scalenohedron, tablets. In addition, different aggregates appear often (grainy, parallel, columnar, basaltiform, lamellar tabular); veins of fibred structure; concretion of zonal addition. Colouring of Calcite is very various - white, reddish, yellow, brown or green.
Calcite in nature often forms dense microcrystalline the masses - limestones which under the action of metamorphism will be transformed in rocks with a saccharoidal structure (granular limestone, marble). The wandering forms of spelaean formations have a fibred structure. They are deposited from waters, rich in a calcspar; typical karst formations (stalactites, stalagmites, curtain etc.) turn out thus, and also oolite, pizilite and typical travertins.
Diagnostic indication.
Many crystals fluoresce in ultraviolet rays red, yellow, rose or dark blue by a color. Often there is thermoluminescense. We will dissolve in muriatic acid. Scratches a knife. From point of optics Calcite finds out property of double refraction. The ray of light, passing through a crystal, fissions on two shining, moving up into a crystalline body with different speed. It creates the phenomenon of double image.
Origin provenance genesis.
Calcite - typical mineral of sedimentary genesis. It appears as a result of the chemical besieging (at evaporation of solutions, enriched a calcspar) or accumulation in sinking of inorganic tailings of marine organisms, which use a calcspar, cut-in in water, for forming of the shells (organogenetic limestones). Calcite can also have a metamorphic origin and, quite rarely, magmatic. Limestone rocks are widespread on-the-spot sew on planets, making 40% its areas, while their gravimetric stake in composition the earth's crust - only 4%.
Deposit minefield mine field occurrence subsoil.
The large, clean and transparent crystals of Calcite are found in emptinesses of Icelandic basalt ragstone (Icelandic spar). The crystals of rose or chlorine color are obtained in Kamberlende (England, EU). Other glorified deposits are Dzhoplin (state of Missouri, USA) and Frayberg (Germany, EU). Druses of crystals very interesting from Fontenblo (France, EU) and state Michigan (USA), including dendrites coppers.
Use, practical application, deployment.
The dense massive forms of Calcite (limestones and granular limestone, marble) are used in building as cement raw material, build stone and finishing material. In chemical industry they are used for the production of caustic soda, anhydrite of carbon, chloride of calcium. In metallurgy they are used as flux. Ground up earthy the masses of Calcite are used as polishing sprinkles of snow, and also at the production of rubber and varnishes. Varieties. On the basis of presence of such components, as a manganese, bivalent iron, zinc, barium, lead and strontium (they partly substitute for a calcium), it is possible to select the row of varieties of Calcite: manganocalcite, ferrocalcite, zincocalcit, cobaltocalcit et cetera.





The river of Kotuy (Middle Siberia, Russia, CIS) is a preserve of stone sphinxes and natural mineralogical museum. The first threshold is Sonata. Fossil corals of different forms and kinds. Fossil mineralogical paradise. Photo, 2014.

Precipices, wholly consisting of single and colonial corals of different kinds and sizes.
Look like horizontal basaltic slates, actually it is ancient corals

A coral precipice is elements of ancient coral fossil reef and fragments of corals.
Look like stone basaltic a separateness and result of activity of volcanos

One of large fossil corals. We such corals were nicknamed by "ears" - structure.

Different types of colonial and single corals are in one fossil standard. Sponges.

Single fossil coral is in the waterside precipice of Kotuya. Flints. Fallen off. Cracks are in a precipice.
Perhaps, one of the most many-sided in the world minerals is a natural calcspar CaCO3 - Calcite. The more so there is not other mineral which, like Calcite, as mountain rocks - limestone, swept marble - would compose multikilometre layers on thousand-kilometre spaces of continents, whole mountain systems. From the same matter coral polypuses created one of the most grandiose buildings on Earth is the Large Barrier reef, stretched out on a 2000 km coast-wise Australia.
Calcite is the whimsical and mysterious world of caves, white-rock radiance of the most ancient cities of the world and not subject millenniums the Egyptian pyramids, marble columns of ancient temples and multicoloured revetment of the stations a subway. But yet more various, the crystals of Calcite look colourfully. They both look like thin needles, both on flat parallelepipeds or on the fragile folias of paper (such Calcite name a paper spar).
The large transparent crystals of Calcite, at the place of one of the first finds of this variety, name the Icelandic spar. In the rhombohedrons of the Icelandic spar, it is possible to sm the interesting optical phenomenon - all, that evidently through them, seems divided. So proves one of surprisings properties of some transparent crystals is a double refraction. To become a jeweller stone his low hardness (3 on Mohs hardness - mineral is one of standards of hardness of this scale) and cleavage does not allow Calcite is ability easily cleave at a blow on symmetric polyhedrons - rhombohedrons. But from other side, exactly low hardness does a fine-grained calcite rock is a marble - by favourite material of sculptors and architects.
Another very notable property of Calcite is his reaction with acids. It is enough to inflict even the drop of weak acid on Calcite, as it begins to hiss, bubble. So him it is easily possible to distinguish from alike minerals. A likeness of Calcite with other minerals is conditioned the variety of his forms and colourings. Calcite can be honey-yellow, purple, green, gently-rose, orange, brown, blue, raspberry. In the long row of admixtures, givings Calcite various colors, iron, manganese, cobalt, nickel, rare-earth elements, is most ordinary. There is a row of minerals, shallow inclusion which are painted by Calcite. So, the blood-red is become by Calcite, containing fine-grained Cinnabar, Vermilion, Spanish Red, the green, blue, dark blue colouring is given Calcite of admixture of carbonates of copper. There is black Calcite - antrakonite, painted including of bitumens.
Calcite - ubiquitous mineral. Seems, there are not such processes on Earth, which it does not appear century There are even magmatic mountain rocks are carbonatites, where Calcite together with other minerals crystallized from thousand-grade fusions. Most various in a due form and flower Calcites arise out of hot underground solutions is a hydrotherm. Everybody can look after certain similarity of this process in a tea-pot: appearing here scum - it that Calcite. At much more low temperature from a that calcspar create buildings, shells, skeletons countless living creatures are shellfishes, corals, sea-urchins and stars. The colonnades of stalactites and stalagmites, whole gloomy world of caves, arise out of cold underground waters.
The remarkable standards of this mineral are found in the great number of places of earth. Meter crystals of the Icelandic spar from Siberia, varicoloured Calcites from Mexico, "stone flowers" from the caves of Middle Asia and kaleidoscope of various crystalline forms from the ore deposits of Seashore - only small stake of the most beautiful standards of Calcite, gettings in hands stone lovers.
Calcite, or lime spar, - third on prevalence in the earth's crust of mineral after a quartz and feldspar. Calcspar. Glance (glitter, glare) glassy. It is transparent, but more frequent opaque. Colors: white, grey, yellow, gold, reddish, brownish, green; it is colourless. A line is white, at strongly muddy differences can be and coloured. Fracture, break padman (more frequent step cleavages on the planes of cleavage). Cleavage very perfect absolute.
If to drip on Calcite by cold dilute muriatic acid, the bubbles of carbon dioxide are intensively selected (Calcite "hisses"). Calcite is build limestones, be found also in sandstones and marls. As cement or component part is in many metamorphic rocks, in hydrothermal ore vein, lode, mines; in emptinesses be found as Druses and wandering formations (for example, forms stalactites in caves).
Crystals (trigonal Crystal structure) are very widespread. In their cutting the verges of sharp rhombohedrons prevail often, and also scalenohedron and prisms. Crystals are rich in combinations of different simply-shaped. Twins are not uncommon. Large crystals appear in cracks and drusy cavities. By a side by side with this short-grained the masses of Calcite are very widespread in nature, aggregates of columnar, basaltiform excretions or coarse-crystalline macrocrystalline block Calcite, cleave on cleavage like feldspars. Be found everywhere.
The Icelandic spar is a colourless aquatic-transparent variety of Calcite with the brightly expressed birefringence of light. Technical application use finds in scopes. Most rich from the known deposits it is already outspent in east part of Iceland; in the CIS be found in trap, traprock of the Siberian platform. Carbonate of calcium is presented the varieties of mineral of Calcite and his polymorphic modification - aragonite.
