Stone, minerals and semiprecious of the world stone
Native elements: Antimony, Stibium -->rus
 Diagnostic cart.
Diagnostic cart.
Sb
Crystal structure trigonal
Hardness on the Mohs scale 3-3,5
Specific unit weight mass 6,7
Cleavage perfect absolute
Fracture, break wrong
Colors white silvery
Colors in powder triturate grey
Glance (glitter, glare) metallic
Now and then observed as individuals of pseudocube or plate tablet lamellar type. More widespread form is grainy the masses, encrustations and concretion of radiant structure. Colors white silvery, brilliance is metallic, very strong. A toxic metallic element that exists in two allotropic forms and occurs principally in stibnite. The stable form is a brittle silvery-white crystalline metal that is added to alloys to increase their strength and hardness and is used in semiconductors. Symbol: Sb; atomic no.: 51; atomic wt.: 121.757; valency: 0, -3, +3, or +5; relative density: 6.691; melting pt.: 630.76oC; boiling pt.: 1587oC.
Ag contains sometimes, Fe or As. Continuous grainy excretions, rarer wandering aggregates (kidney-shaped reniform, cluster-like), sometimes radiant structure; crystals are rare. Structure and morphism crystal. Trig. p. D53d-R3m; arh = 4,507 A; a= 57 6o 6o06; Z = 2; ah = 4,310; ch = 11,318 A; ah : ch = 1 : 2,627; Z = 6. Structure of type of arsenic. Distances of Sb-Sb 2,87 and 3,37 And. Ditrigon-skalenoedr. class; and : with = 1 : 1,3236 Crystals rhombohedral, thickplate tablet lamellar to on (0001) or lamellar tabular. Dv. to on (1012); form difficult groups - four, six, often polysynthetic. Cleavage on (0001) perfect absolute, to on (2021) sometimes clear, to on (1120) and on (1012) noperfect absolute.
Diagnostic indication.
Low hardness (scratches a penknife). In acids does not dissolve, burns on air, selecting white smoke. Knows the antimony (British Antimony, French Antimoine, German Antimon) of persons since olden times both as a metal and as some compounds.
Origin provenance genesis.
Antimony, Stibium has a hydatogenesis usually. Appears during hardening of remaining fluid in the process of crystallization of granites and pegmatites. Therefore be found in hydrothermal vein, lode, mines jointly with difficult sulfoarsenate and sulfoantimoniate, mainly such metals, as silver.
Deposit minefield mine and use.
Found out the two-bits of this mineral in Saravake, on Borneo, in Sala (Sweden), in Andreasberge (Germany), in Koimbre (Portugal), in Canada and California. For the industrial use an antimony is extracted from its salts. A native metal presents scientific and collection interest only.

Antimony, Stibium. Dauphin, France. A photo: © A.A. Evseev.


Antimony, Stibium. Seynyayoki, Finland. Antimony, Stibium. Burkandya, Magadan, Russia. A photo: © A.A. Evseev.
Antimony, Stibium - on a slang "vomitive emetic stone". Antimony, Stibium gets in an organism with food and preferentially concentrated in a thyroid, hepar, spleen, skeleton, buds, to blood (in red corpuscles) and in other soft organs and tissues of man. Antimony accumulates in red corpuscles, Stibium in the degree of oxidization +3, in plasma of blood (lymphocyte) - in the degree of oxidization +5. From the organism of Antimony, Stibium hatches slowly, with urine (to 80%).
Maximum possible concentration of antimony in the organism of man - 10-5-10-7 g on 100 g of dry mass of tissues. During the high concentration of Antimony, Stibium inactivate the row of enzymes of lipidic, carbohydrate and albuminous exchange (as a result of blocking of sulfhydryl groups, red Cinnabar replaces, Vermilion, Spanish Red is a dry sulfide of mercury of red color).
At the reception of preparations of antimony inward the sharp or chronic poisoning can develop in toxic doses. Antimony, Stibium forms compounds with atoms grey (reacts with the sulfhydryl (thiols) groups of enzymes), that stipulates its high toxicness (Antimonite). Basic displays of surplus of antimony: loss of appetite, inflammation of mucous membranes of pharynx and larynx, dryness in a throat, nausea, vomiting, pains in an intestine, increase and sickliness of hepar, icteritiousness sclera; protracted cough.
General action of preparations of antimony like the action of arsenic. Antimony, Stibium has more sharp local action, causing the cruel vomiting of reflex origin, heavy anatomic defeats of gastroenteric highway; Stibium preparations are more difficult sucked in, and poisoning flows slower. A fatty regeneration and cirrhosis of parenchymatous organs, hepar and buds develops at the use of preparations of antimony. The considerable toxicness limited application of preparations of antimony in medical aims as self-treatment (psychiatry of brain).
Long time such compounds of antimony, as fivesulphureous Antimony, Stibium, wines-antimonial-potassium ("vomitive stone") and wines-antimonial-sodium of salt was used as coughings up and vomitive facilities. To cause vomiting, wine, self-possessed in a antimonial vessel, was given a patient. And, in addition, Antimony, Stibium replaces red Cinnabar, Vermilion, Spanish Red (poisonous sulfide of mercury) at treatment of psychiatric disorders and hallucinations - under an electroshock (electrodes in a brain). At the beginning of ХХ century compounds of antimony applied also as coughings up and antiparasitogenic facilities.
The oxide of antimony (III) in ancient Greece served for treatment of skin illnesses, and in middle ages used as therapy of leprosy, syphilis and cardiac diseases. In modern medicine preparations of antimony (solusurmine and other) are used for treatment of row of infectious diseases of man and animals (pathologies of brain, as well as red Cinnabar, Vermilion, Spanish Red is a sulfide of mercury, substitutes for each other). They are used for treatment of visceral and skin leishmaniasis, African kaodzera (to the sleeping-sickness), at researches of hemopexis (on red Cinnabar, Vermilion, Spanish Red, in the complex of electro-shock therapy). Food sources of antimony: marine products seafood, green-stuffs, fruit, juices, drinks.


Antimonite. Sikoku., Japan. Brush of pinacoidal of prismatic crystals. Baya-sprie, Romania. A photo: © A.A. Evseev.
Dangerous "hazardous cargo", signs #6.1 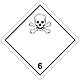
Toxic non biological matters substances (toxin)
Risk of poisoning at inhalation, contact with a derma or swallowing. Make a danger for a water environment or sewage system
To use a mask for emergency abandonment of transport vehicle
White rhombus, number of Dangerous "hazardous cargo", signs, black skull and crossed cross-bones
Dangerous "hazardous cargo", signs #8 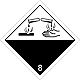
Corrosive (caustic, pungent, acrid) substances matters
Risk of burns as a result of eating away of derma. Can stormily react between itself (components), with water and other matters. Matter, that spilled / scattered, can select a corrosive pair.
Make a danger for a water environment or sewage system
White overhead half of rhombus, black - lower, isometric, number of DOPOG, test tubes, hands
