Stone, minerals and semiprecious of the world stone
Sulphide: Sphalerite (zinc-blende) -->rus
 Diagnostic cart.
Diagnostic cart.
Two standards of sphalerite, plugged in a carbonate rock
Zn S (zinc-sulfide)
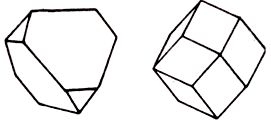
Crystal structure cube
Hardness on the Mohs scale 3,5-4
Specific unit weight mass 3,9-4,2
Cleavage perfect absolute
Colors colourless, various
Colors in powder triturate whitish, brownish
Glance (glitter, glare) from resin to semimetallic
Sphalerite (zinc-blende), is a remulin. Glance (glitter, glare) diamond, rarer metalloid, dim, in a fracture fat. Colouring is honey-yellow, brown to black (depends on maintenance of iron). Skorlupovataya blende is accretions of sphalerite with a wurtzite (hexagonal modification of ZnS). A line is yellow to by a borax. Fracture, break step. Fragile. Cleavage perfect absolute. A yellow to brownish-black mineral consisting of zinc sulphide in cubic crystalline form with varying amounts of iron, manganese, cadmium, gallium, and indium: the chief source of zinc. Formula: ZnS Also called: zinc blende. From Greek sphaleros deceitful, from sphallein to cause to stumble.
Be found mainly in hydrothermal, and also in basic sediments deposits. Crystals (cube Crystal structure) are usually distorted. Polisinteticheskoe double condition shading on verges. Usually composes thin- and coarse-grained aggregates with the well expressed planes of cleavage. Main base ore of zinc. Places of distribution: Germany, Sweden, Spain, CIS, USA.
 Besides zinc and sulphur, sphalerite (him other name is a zinc-blende) can contain many other elements. Among them is iron (limit 20% is a variety of marmatite ) manganese, cadmium, sometimes also indium and gallium (Ga). Crystallized in cube Crystal structure, mainly as tetrahedrons and rhombic-dodecahedron shape (diamond forms). Sometimes it is difficult forms, often with stroke and bent verges. There are also continuous the masses - grainy, fibred and kidney-shaped reniform. Colors of sphalerite is very changeable, from colourless to black, through brown tones - to yellow, rose, green. Colors of powder is varied from white to brownish; brilliance from resin to diamond, up to almost metallic at a marmatite. A mineral can be transparent, translucent, and also opaque, if contains a lot of iron.
Besides zinc and sulphur, sphalerite (him other name is a zinc-blende) can contain many other elements. Among them is iron (limit 20% is a variety of marmatite ) manganese, cadmium, sometimes also indium and gallium (Ga). Crystallized in cube Crystal structure, mainly as tetrahedrons and rhombic-dodecahedron shape (diamond forms). Sometimes it is difficult forms, often with stroke and bent verges. There are also continuous the masses - grainy, fibred and kidney-shaped reniform. Colors of sphalerite is very changeable, from colourless to black, through brown tones - to yellow, rose, green. Colors of powder is varied from white to brownish; brilliance from resin to diamond, up to almost metallic at a marmatite. A mineral can be transparent, translucent, and also opaque, if contains a lot of iron.
Chemical composition (chemistry, compound) (in %): Zn- 67,1; S - 32,9; from admixtures iron (more than 25% Fe) is most characteristic, rarer present is a cadmium, gallium, manganese, Mercury, Quicksilver and other Geksatetraedricheskiy type of symmetry. Cleavage - perfect absolute on rhombic-dodecahedron shape (diamond forms) (110). Be found in crystals tetrahedron, rarer than rhombo-dodecahedral habitus. Main simple forms: (111), (101), (100), (110), (113), (112), (122) and other On verges often there are shading, stages and spirals of growth. Twins are not uncommon to on (111), including polysynthetic, noticeable on the parallel shading on the planes of cleavage. Continuous the masses of sphalerite are widespread. The kidney-shaped reniform cryptocrystalline aggregates of concentric-zonal structure, stalactites, are rarer marked. All-epitaxial joint, junction with chalcopyrite and paramorphism is known on a wurtzite.
Diagnostic indication.
There are varieties adamantine, heavy with perfect cleavage, with the fracture of type of padman. Some varieties show triboluminescence (luminescence at mechanical influences). There is also fluorescence of rose color in ultraviolet rays.
Origin provenance genesis.
Sphalerite - widespread mineral. It appears in various geological terms. Discovered, for example, in hydrothermal deposits, both high- and low temperature, where often associated with galena, galenite, chalcopyrite, marcasites, Pyrite and gangue from a quartz, barytes and Fluorite. It be found also in limestones, affected hydrothermal processes.

Deposit minefield mine and use.
Large deposits are found in Germany, Romania, Spain, France, Sweden, England, Scotland, Japan, Australia, Mexico, USA. There is the most considerable deposit (foremost from point of well well-educated crystals) in Italy - Bottino, in a neighborhood of Serravecca, in Paduanskikh Alps. From here the excellent standards of sphalerite acted in an association with chalcopyrite, Meneghiniteом, jamesonite, galena, galenite et cetera Crystals of unsurpassed beauty, perfect form and color find in geode in granular limestone, marble Karrary and in the dolomites of Valle-di-binn in Valeze. Sphalerite is the most essential ore for the receipt preparation of zinc, and also rare dissipated elements: cadmium, gallium and indium, In.





Glance (glitter, glare) diamond. Yantarno-yellow, gold sphalerite is named also honey blende, orange-red - ruby blende. Accepts a polish hardly - perfect absolute cleavage hinders in three directions. Varieties, suitable for cutting, meet in Spain (Santander) and in Mexico (Chivor). Entangling sphalerite is possible with many yellow jeweller stone, and colourless - even with a diamond.

Sphalerite, Quartz. Dalnegorsk, Seashore, Russia, CIS. A photo: © A.A. Evseev.

Sphalerite, Sferolitovyy, kidney-shaped reniform, rhythmic aggregate, with galena, galenite.
Raybl, Friuli - Venice -Julia, Italy (ES). A photo: © A.A. Evseev.

Sphalerite (wurtzite). Beregovo, Zakarpate, Ukraine (CIS). A photo: © A.A. Evseev.


Sphalerite (kleyophane). Spaynyy pricking out. Pikos-de-europa, Santander, Spain (ES). A photo: © A.A. Evseev.
Biological role. Zinc is needed for functioning of DNA- and RNA-polimeraz, supervisory the processes of passing to the inherited information and biosynthesis of albumens and reparative processes in an organism; and also enzyme of key reaction of biosynthesis of hema which is included in the structure of haemoglobin, histohematins of respiratory chains of mitochondrion, mitochondrium, histohematin of R-450, catalase and myeloperoxidase. Zinc is included in the structure of key antioxidant, antioxidative enzyme - (Zn, Cu) - superoxide dismutase - and induce the biosynthesis of protective albumens of cage - metallothionein, - zinc is the antioxidant of reparative action.
Signs of insufficiency. Loss of feelings of taste and smell; fragility, removing a layer by the layer of nails, formation on them of white spots; acne; delay of pubescence; fatigue; deceleration of growth, psilosis; enhanceable level of cholesterol; weakening of sharpness of scotopia; fruitlessness, impotence, violation of functions of prostatic gland; enhanceable susceptibility to the infections; weakening of memory; predisposition to diabetes; skin defeats and slow cicatrization of wounds. The table of contents of zinc goes down at the chronic diseases of hepar and buds, at presence of tumours, burns and at the heart attack of myocardium.
Basic displays of surplus of zinc: violations of functions of the immune system, autoimmune reactions; violations of the state of skin, hairs, nails; sickly sensitiveness of stomach, nausea; decline of maintenance in the organism of iron, copper, cadmium; weakening of functions of prostatic gland; weakening of functions of pancreas; weakening of functions of hepar. Content of zinc rises at anaemias, leukemia, atherosclerosis, hypertensive illness, hyperthyroidism, overstrain, stresses. Nails - without white spots.
Zinc is needed: for the prophylaxis of blackhead, comedo, at prostatitis, impotence, lipidemia. Day's requirement of masculine organism more than for women in zinc, and makes 15-25 mgs. Suction of zinc takes a place in a thin bowel. A main way of egestion is a gastroenteric highway. The amount of zinc, secreted in a bowel, changes depending on his consumption. About 400-600 a mcg is selected daily with urine. Zinc is lost with sperm and menstrual secret. The states of catabolism (burns, operations, traumas, starvation) result in the clinically meaningful increase of losses of zinc with urine. Superficial losses by means of desquamation (peeling-offs) of skin, with growth of hairs and with then make to 1 mgs/days. In an initial period of pubescence, when privy parts are formed, the enhanceable amount of zinc is required boys. Zinc needs the organism of girls in this period in less - for general growth and development. For grown people men on a background the deficit of zinc there can be a decline of impregnating ability of spermatozoa. All You need is love ...
Zinc is needed for the synthesis of albumen, including collogen, wound - and ulcer, sore- cicatrize action effects possesses therefore, participates in the processes of taste perception and sense of smell, needed for functioning of cns, including - for the processes of memorizing. Zinc is vitally important for functioning of thymus and normal state of the immune system of organism. Being, besides, by the component of retinol-transport albumen, zinc together with the vitamin of A (and by the vitamin of C) hinders the origin of immunodeficiency aphylaxis (AIDS), stimulating the synthesis of antibodies and rendering an antiviral action effects. Zinc is regulated by the level of metabolite of testosterone (orchidic hormone) - dihydrotestosterone, surplus of which stipulates hyperplasia of prostate. Zinc is a necessary factor and for a womanish organism, because included in the structure of receptors for estrogens, regulating all of oestrogen dependent processes thus.
Zinc is required for maintenance of normal concentration of vitamin of E in blood, it increases absorption of vitamin of E. Cink irreplaceable for normal growth and development, therefore a requirement in zinc is increased during pregnancy. This necessity is small: 100 mgs on all of period of pregnancy. It is especially important to provide a requirement in zinc of fruit in the first 3 months of pregnancy. Development of placenta in this period requires large mineral supplies. An expectant mother grumbles about changes in perception of smell and taste. It is a result of lack of zinc in the taste papillae of language or in the receptors of nasal cavity. The deficit of zinc for expectant mothers can result in the atonic bleeding, premature births, protracted childbirth parent process (birth).
There is information, that gallium (component of sphalerite) is included in the shells of red corpuscles of blood and hinders formation of blood clots. Mainly gallium enters organism with food and contained in tissues in negligible quantities (0,01-0,06 mcg/g). There is information, testifying to being of gallium in ductless glands, in particular, in a hypophysis. A "depot" of gallium is bone tissues and hepar. Basic displays of surplus of gallium: there is a defeat of the nervous system at poisoning, that is accompanied morphological changes in a hepar and buds. There are considerable vibrations in maintenance of potassium and natrium sodium in the whey of blood, damage of mucous membranes of gastroenteric highway (caustic, eats away tissues).
Use of gaas (production of semiconductors) from the beginning of 80th XX century resulted in the increase of risk of intoxication this element of not only workers of electronic industry but also population. By a basic "target" for a gaas (poisonous compounds of arsenic - type sulfide) there is the immune system in an organism. This element is able to violate formation of gels (a silica is opals in an organism), due to strengthening of egestion of aminolevulinic acid and porphyrinic.
Gallium is needed: in medicine nitrate of gallium is used for treatment of hypercalcemia for oncologic patients, where the effect of action is arrived at due to suppression of activity of osteoclast, osteoclastus. The radioisotopes of gallium are applied in diagnostics and oncotherapy. Gallium (in itself) comparatively is little toxic. Possibility of application of gallium is studied for treatment of chronic infectious diseases of lights (including, possibly, and tuberculosis) and cystophorous fibrosis (mucoviscidosis, fibrocystic disease (cystic fibrosis) of pancreas). Food sources of gallium: wheat groats (especially - manna), honey, many types of mushrooms (including are Boletus edulis cepe - white fungus mushroom).
DOPOG #4.1 
Flammable hard solid substance matters, self-reactive matters and solid desensitized explosives
Risk of fire. Flammable or combustible matters can catch a fire from sparks or flame. Can contain self-reactive matters, apt at exothermic decomposition in the case of heating, contact with other matters (such as: acids, connections of heavy metals or amines), to the friction or blow.
It can result in the selection of harmful or flammable gases or pair or spontaneous combustion. Capacities can burst at heating (dangerous, perilous - does not burn practically).
Risk of explosion of the desensitized explosives after the loss of desensitizer
Seven vertical red striaes on a white background, isometric, number of DOPOG, black flame
DOPOG #4.3 

Matters substances, which select flammable gases at a contact with water
Risk of fire and explosion at a contact with water.
Gruz, which had scattered, it is necessary to cover and hold dry
Sine-blue rhombus, number of DOPOG, black or white flame
DOPOG #5.1 
Matters substances, which oxidize
Risk of stormy reaction, self-ignition or explosion at a contact with combustible or flammable matters
To shut out formation of mixture of load with flammable or combustible matters (for example by sawdusts)
Yellow rhombus, number of DOPOG, black flame above by a circle
DOPOG #6.1 
Toxic non biological matters substances (toxin)
Risk of poisoning at inhalation, contact with a derma or swallowing. Make a danger for a water environment or sewage system
To use a mask for emergency abandonment of transport vehicle
White rhombus, number of DOPOG, black skull and crossed cross-bones
DOPOG #8 
Corrosive (caustic, pungent, acrid) substances matters
Risk of burns as a result of eating away of derma. Can stormily react between itself (components), with water and other matters. Matter, that spilled / scattered, can select a corrosive pair.
Make a danger for a water environment or sewage system
White overhead half of rhombus, black - lower, isometric, number of DOPOG, test tubes, hands
DOPOG #9 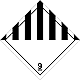
Other hazardous substances matters and produce
Risk of burns. Risk of fire. Risk of explosion.
Make a danger for a water environment or sewage system
Seven vertical black striaes on a white background is a top, white - lower half of rhombus, number of DOPOG
