Stone, minerals and semiprecious of the world stone
Sulphate: Gypsum -->rus
 Diagnostic cart.
Diagnostic cart.
Ca SO4 * 2 (H2O) hydrite
Crystal structure monoclinic
Hardness on the Mohs scale 2
Specific unit weight mass 2,3-2,4
Cleavage perfect absolute
Fracture, break wrong
Colors colourless, multicolors
Colors in powder triturate white
Glance (glitter, glare) from glass to mother-of-pearl
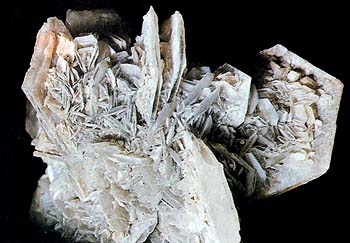
Gypsum - the name behaves both to the mineral and to the consisting of him rock. Vodosoderzhaschiy sulfate of calcium. Glance (glitter, glare) glassy to mother-of-pearl. A line is white. Fracture, break uneven, flexible, but not elastic. Cleavage very perfect absolute. Appears in an association with rock-salt and anhydrite. Crystals (monoclinic Crystal structure) are not uncommon, more frequent all of plate tablet lamellar, prolate or needle (fibred). Cruciform twins of germination or twinning joint, junction are ordinary in a kind "dovetail". In areas joint, junction, collected a wall outlet - "roses of the desert meet with a droughty climate". Deposit minefield mine field occurrence subsoil: Tyuringenskiy the Forest, Garc (Germany), Karintiya (Austria), Switzerland, Italy, France, Chile.
Alabaster - dense white or granular limestone, marble-like variety of gypsum (a fine-grained usually white, opaque, or translucent variety of gypsum used for statues, vases, etc, a variety of hard semitranslucent calcite, often banded like marble, of or resembling alabaster). The known deposit is in Toskane, Italy. Gypsum is a colourless or white mineral sometimes tinted by impurities, found in beds as an evaporite. It is used in the manufacture of plaster of Paris, cement, paint, school chalk, glass, and fertilizer. Composition: hydrated calcium sulphate. Formula: CaSO4 * 2H2O. Crystal structure: monoclinic. Anhydrite - a colourless or greyish-white mineral, found in sedimentary rocks. It is used in the manufacture of cement, fertilizers, and chemicals. Composition: anhydrous calcium sulphate. Formula: CaSO4. Crystal structure: orthorhombic.
 Clean pure gypsum - colourless mineral; but the presence of including and presence of elements-admixtures can condition various colouring. For a gypsum twins are characteristic in a kind dovetail and spearhead.
Clean pure gypsum - colourless mineral; but the presence of including and presence of elements-admixtures can condition various colouring. For a gypsum twins are characteristic in a kind dovetail and spearhead.
Crystals of gypsum mainly white, but can have also grey, rather yellow and other colouring, conditioned including or elements-admixtures. Aggregates are widespread, such as Selenite (gypsum) are crystals or wide plates; serikolite -fibrous accumulations of the extended crystals; an alabaster is grainy the masses of dense addition, sometimes zonal, look like a beeswax. A gypsum is soft, has perfect cleavage, divided into thin plates and scales.
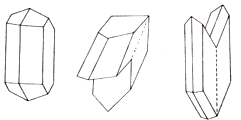
Chemical composition (chemistry, compound). Kemidol (SAO) 32,6%, trioxide 46,5% is grey (SO3), water (N2o) 20,9%. Form of crystals. Tablitchatye, prismatic, columnar, basaltiform, needle; frequent twins of germination on one of two laws:
1) twins dovetail, using most proliferation-paired twinning (duplex) on the verges of prism;
2) monmartrs (parisian) twins-ribs of prisms are located parallell to the twinning guy-sutures.
Crystalline structure. Stratified. Class of symmetry. Prismatic - 2/t. Cleavage. Perfect, parallel lateral verges (010); fracture on the verges of prism thinly rough, and on basale verges - distinct padman. Aggregates. Dense, grainy (alabaster), fibred, scaly, earthy, concretions.
Diagnostic indication.
Will dissolve a mineral in muriatic acid and in hot water; paints flame of blowpipe in a rose-red color; can luminesce in ultraviolet rays. Decomposes with the loss of crystallizational water and melt fuse in a white enamel. In the closed kimberlite tube loses crystallizational water, growing into the sulfate of calcium ("firmly burnt gypsum"). A behavior is in acids. Dissolves poorly.
Origin provenance genesis.
A gypsum is the typical mineral of evaporite origin. He, however, can appear and during hydratation of anhydrite, besieged from gases of fumarole (a sublimation is a transition from gaseous in the hard state). In addition, a gypsum is formed at affecting of water sulfides of metals. It is often associated with native sulphur, especially in the so-called gypsum-sulfur-containing structure - thicker than rocks the outputs of which border the external arc of Apennines and closed in Sicily.
Deposit minefield mine field occurrence subsoil.
The gypsums of Paveze are well known in Italy, болонских Apennines and Sicily. Giant crystals act from here. Very beautiful copies are found in Mexico, Chile and United States. The beds of gypsum are developed in industrial scales in the Parisian pool (France), in Canada, in many districts of the United States and Russia. In Italy found out them in Vol'terre (a province Piza), where an alabaster is obtained.
Finally, the so-called "roses of the desert" are widely known are accretions of gypsum as a wall outlet; colouring pinkish-yellow. "Rosa of the desert" take a place from the deserted areas of Morocco and Tunis (Africa) and states Arizona and New Mexico (USA). On a photo: characteristic "rose of the desert" from lamellar tabular crystals from Algeria. There are such interesting formations of gypsum and in Russia.



Use, practical application, deployment.
Thinly ground and partly water-free, and then the pressed gypsum is used on build business as astringent material, and also as a component of Portland cement and build mixtures. Due to the low hardness a gypsum is used for creation of sculpture and in a decorative art. Some varieties of alabaster find application use as finishing material.
