Stone, minerals and semiprecious of the world stone
Sulphide: Galena, galenite -->rus
 Diagnostic cart.
Diagnostic cart.
Two standards of galena, galenite: above - cuboctahedron from the mine of Seravecca Lukka; down - cube type in a dolomite (Joplin, Missouri, USA)
Pb S (sulfide of lead)
Crystal structure cube
Hardness on the Mohs scale 2,5-3
Specific unit weight mass 7,2-7,6
Cleavage perfect absolute
Fracture, break semipadman
Colors leaden-grey
Colors in powder triturate blackish-grey
Glance (glitter, glare) metallic
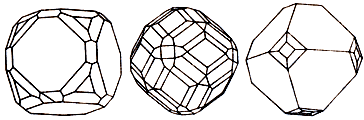
 Galena, galenite (galena), is a sulfide of lead. Strong metallic brilliance. Opaque. Colors leaden-grey, sometimes with dark blue or reddish oxide tint. A line is darkly-grey to black. Fracture, break step. Fragile. Cleavage very perfect absolute. Be found in hydrothermal ore deposits in an association with sphalerite, in stratoforms (stratosphere) deposits in form beds or sprinkled ores. Crystals (cube Crystal structure) are presented cubes, octahedrons or their combinations.
Galena, galenite (galena), is a sulfide of lead. Strong metallic brilliance. Opaque. Colors leaden-grey, sometimes with dark blue or reddish oxide tint. A line is darkly-grey to black. Fracture, break step. Fragile. Cleavage very perfect absolute. Be found in hydrothermal ore deposits in an association with sphalerite, in stratoforms (stratosphere) deposits in form beds or sprinkled ores. Crystals (cube Crystal structure) are presented cubes, octahedrons or their combinations.
Usually in continuous dense the masses, from rough - to short-grained. Main base and the most widespread leaden ore. The basic booty of silver is also related to his passing extraction from galena, galenite.
Crystallized in cube forms, with a characteristic leaden-black color. Opaque, with metallic brilliance, perfect cleavage on the verges of cube. More frequently there are the masses of dense or grainy addition. They differ strong brilliance on-the-spot fresh fracture, in course of time nigrescent.
Origin provenance genesis.
Deposit of minefield mine field occurrence subsoil galena, galenite on the whole have a magmatic origin. Appears in hydrothermal vein, lode, mines, formed as a result of consolidation of remaining solutions which appear at forming of such rocks, as granites and pegmatites. In these cases mineral is in an association with sphalerite, vitreous silver, quartz and Fluorite. Galena, galenite, in addition, can appear and in basic sediments terms. It is considered that such deposits appear due to the concentration of minerals, originally dissipated in a rock. The large beds of galena, galenite can have such origin in limestones and dolomites.

Deposit minefield mine and use.
Very rich deposits in America are dissipated mineralization. They are in three states - Missouri, Oklahoma, Kansas. This district is named by Tri-stayt ("three states"), and the most famous center in him is Joplin (st. Missouri, USA). The Australian (Broken-khill), English (Kamberlend), mexican (Eulaliya) and German (Andreasberg and Frayberg) deposits are widely known also. In Italy there are the deposits developed during many centuries - it Raybl (Karniyskie Alps), Mountain Dossena (foot-hills of Alps are in Lombardy), Monteponi and Montevekk'o (Sardinia). Galena, galenite - main ore mineral lead. From some silvercontained galenite extract silver as by-product.


Galena, galenite in concretion of phosphorite. Kamenec-Podolskiy, Ukraine (CIS). A photo: © A.A. Evseev.

A.A. Evseev.

Galenite of amazon-stone pegmatites. Flat m., Keyvy, Kola peninsula, Russia (CIS)). A photo: © A.A. Evseev.

Galena, galenite. Aggregate, squeezed out at tectonic motion of sial in a drusy cavity
through opening between the crystals of quartz. Berezovsk, Sr. Ural, Russian Federation (CIS). A photo: © A.A. Evseev.
Lead is a carcinogen and teratogen for the organism of people. It is vitally a necessity, as influences on the synthesis of albumen, power balance of cage and its genetic vehicle, participates in the exchange processes of bone tissues and gonads. In a norm in bones (cartilaginous and soft tissues) maintenance of lead 20 mgs/kg, in a hepar is a 1 mg/kg, buds are 0,8 mgs/kg, cerebrum are 0,1 mgs/kg. For men (gonads) maintenance of lead in an organism is higher, than for women.
There are 2 mgs of lead in the organism of people. 5-10% is sucked in in a gastroenteric highway (and to 50%). A degree depends on solubility of his compounds. Plenty of lead can get in an organism with respirable air (a to 70% aerosol, containing lead, settles in lights). At the large concentrations of tetraethyllead there is a risk of his penetration through a skin. Lead hatches from an organism with an excrement (80-90%), and less part with urine.
The deficit of lead in an experiment reduces growth of animals, violates metabolism of iron, changes the action of some enzymes and concentration of separate metabolite in a hepar, related to status of iron. Lead is increased by growth and improves hematocrit, packed cell volume, PCV and concentration of haemoglobin at the deficit of iron for rats, this effect was the result of pharmacological action of lead. Mechanism by which lead influences on metabolism of iron - catalyst. Lead is needed: at lowered hematocrit, packed cell volume, PCV and haemoglobin, at the iron-deficient states.
For all of regions of Ukraine and Russia (CIS) lead is an anthropogenic toxic element from the group of heavy (high-density) metals. It is related to high contamination and extrass of exhaust-gass of motor transport, working on the ethylated petrol. A from 5% to 30% population in cities suffer from surplus of lead. During sharp intoxication lead neurological symptoms, leaden encephalopathy, cerebropathy, "leaden" colic, nausea, locks, pains on all of body, decline of frequency of heart-throbs and increase of arteriotony, are marked. During chronic intoxication there is a hypererethism, hyperactivity (violation of concentration of attention), depression, decline of IQ (index of estimation of intellect), high blood pressure, peripheral neuropathy, neuritis, loss or decline of appetite, pains in a stomach, anaemia, nephropathy, "leaden border", dystrophy of muscles of racemes of hands et cetera
Displays of surplus of lead: hypererethism, weakness, fatigueability, decline of memory; head pains; defeats of the peripheral nervous system (pains are in extremities); appearance of leaden border on gum; decay of teeth, arthropathy, arthropathia, diseases of the bone system, increase of arteriotony, development of atherosclerosis, stomach-aches (leaden colics), spastic, spasmodic locks; exhaustion, weight loss, malnutrition, decline of mass of body, violations of porphyrinic exchange (urobilinogen, coproporphyrin), nephropathy, making progress kidney insufficiency worsening of mobility of spermatozoa and capacity for an impregnation, decline of potency, clasmocytoma, increase of amount of red corpuscles with a basophilous (basophilic) grittiness, anaemia, decline of stability to the infections (especially for children), development of syndrome of saturnism (lead poisoning), decline of maintenance in the organism of calcium, zinc, selenium.
- During the concentration of lead for children violations of development register in blood of 10-20 mkg/100 ml.
- During the proper concentration from 20 to 40 mkg/100 ml is observed decline of IQ.
- During the concentration of lead in blood at adults and children from 40 to 60 mkg/100 ml is marked anaemia and peripheral neuropathy, neuritis.
- During the concentration of lead in blood grown people from 60-80 mkg/100 ml has chronic nephropathy.
- During the proper concentration from 80-100 mkg/100 of ml sharp nephropathy is marked.
- During a concentration from 100-120 mkg/100 of ml is encephalopathy, cerebropathy.
The toxic action of lead is conditioned ability to form compounds with the large number of anions - ligands, which sulfhydryl groups, derivates of cystein, imidazole and carboxyl groups, phosphates, behave to. As a result of binding of ангидридов to lead the synthesis of albumens and activity of enzymes is repressed, for example adenosine triphosphate, ATP. Lead is violated by the synthesis of hema and hematohistone, interfering in a porphyrinic exchange, induce the defects of membranes of red corpuscles. On a background the deficit of iron, calcium, phosphorus, magnesium and zinc ability of organism to master lead is increased. Sulfur-containing amino acid, vitamins of A, C, E, groups of B, folacin, Nicotinamidum, calcium, magnesium, zinc, iron, chrome, phosphorus, selenium instrumental in the decline of level of lead in an organism.
 Dangerous "hazardous cargo", signs #1
Dangerous "hazardous cargo", signs #1 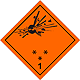
Bomb which explode bursts
Can be characterized in a number of properties and effects, such as: by critical mass; by variation of fragments; intensive fire/thermal heat stream; bright flash; loud noise or smoke.
Sensitiveness to the shoves and/or shots and/or heat
To use shelter, here to repose on safe distance from windows
Orange sign, image of bomb at an explosion
Dangerous "hazardous cargo", signs #6.1 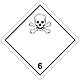
Toxic non biological matters substances (toxin)
Risk of poisoning at inhalation, contact with a derma or swallowing. Make a danger for a water environment or sewage system
To use a mask for emergency abandonment of transport vehicle
White rhombus, number of Dangerous "hazardous cargo", signs, black skull and crossed cross-bones
Dangerous "hazardous cargo", signs #5.1 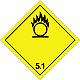
Matters substances, which oxidize
Risk of stormy reaction, self-ignition or explosion at a contact with combustible or flammable matters
To shut out formation of mixture of load with flammable or combustible matters (for example by sawdusts)
Yellow rhombus, number of Dangerous "hazardous cargo", signs, black flame above by a circle
Dangerous "hazardous cargo", signs #4.1 
Flammable hard solid substance matters, self-reactive matters and solid desensitized explosives
Risk of fire. Flammable or combustible matters can catch a fire from sparks or flame. Can contain self-reactive matters, apt at exothermic decomposition in the case of heating, contact with other matters (such as: acids, connections of heavy metals or amines), to the friction or blow.
It can result in the selection of harmful or flammable gases or pair or spontaneous combustion. Capacities can burst at heating (dangerous, perilous - does not burn practically).
Risk of explosion of the desensitized explosives after the loss of desensitizer
Seven vertical red striaes on a white background, isometric, number of Dangerous "hazardous cargo", signs, black flame
Dangerous "hazardous cargo", signs #8 
Corrosive (caustic, pungent, acrid) substances matters
Risk of burns as a result of eating away of derma. Can stormily react between itself (components), with water and other matters. Matter, that spilled / scattered, can select a corrosive pair.
Make a danger for a water environment or sewage system
White overhead half of rhombus, black - lower, isometric, number of DOPOG, test tubes, hands
