Stone, minerals and semiprecious of the world stone
Halogenoide, halogenide, halogen: Fluorite -->rus
 Diagnostic cart.
Diagnostic cart.
Standards demonstrate wonderful crystallize mineral (on a photo: Fluorite - mineral of violet color).
Ca F2
Crystal structure cube
Hardness on the Mohs scale 4
Specific unit weight mass 3,1-3,2
Cleavage perfect absolute
Fracture, break wrong
Colors colourless разноокра-шенный
Colors in powder triturate white
Glance (glitter, glare) glassy
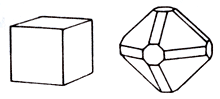
Fluorite is a fluor-spar - fluoride (fluorine) of calcium. Glance (glitter, glare) glassy. Transparent, except for the intensively painted varieties. Colors: violet, dark blue, blue, black, yellow, gold, green, pink, rose; rarer colourless. A line is white. Fracture, break step, even to the padman. Fragile. Cleavage, very perfect absolute on octahedron, fluorescent. Appears in magmatic and basic sediments rocks, and also in hydrothermal terms. The well well-educated crystals of cube Crystal structure are rare. They are presented more frequent than all by cubes, octahedrons or their combinations. Often there are dense grainy or pole aggregates. Widespread mineral of hydrothermal vein, lode, mines and metasomatism beds, crystallized in emptinesses lived pegmatites. One of basic metallurgical gumboils, facilitating smelting of metals from ores.
Fluorite is a white or colourless mineral sometimes fluorescent and often tinted by impurities, found in veins and as deposits from hot gases. It is used in the manufacture of glass, enamel, and jewellery, and is the chief ore of fluorine. Composition: calcium fluoride. Formula: CaF2. Crystal structure: cubic Also called (in Britain and certain other countries): fluorspar, fluor.
Fluorite is presented cube and octahedral crystals, sometimes largeness. There is perfect absolute cleavage on octahedron. Twins are not rare with the mutual germination of crystals. Often there are dense the masses, earthy and concretionary formations. Colors is changeable: clean Fluorite is colourless and transparent, but mineral is yellow, red, green, dark blue, almost black. Colors of line nevertheless always white. Glance (glitter, glare) of Fluorite of glassy, easily scratches a knife-blade; on weight it rather heavy. Flyuoresciruet in ultraviolet rays violet-розовым light.
Chemical composition (chemistry, compound) is maintenance (в%): Sa - 51,2; F - 38,8; the admixtures of chlorine are marked, iron, rare earths, sometimes uranium and other Hexaoctahedral type of symmetry. Cleavage - perfect absolute to on (111). Be found as well well-educated cube, rarer octahedral and rhombic-dodecahedron shape (diamond forms) crystals measuring 3-5 sm, sometimes to 20 sm and even more From other simply-shaped of frequent verge (012), (013), (113), (112), (123) and other Verges of cube usually smooth, and octahedron - mat. Twins of germination are ordinary on a Fluorite law, pole aggregates are widespread, and also continuous grainy, triturate or earthy (ratovkite) the masses
Diagnostic indication.
As at all of minerals, containing a calcium, the color of gas flame has the characteristic red-orange glimmered style. On hardness, easily differs cleavage and type of crystals of Fluorite from other minerals of calcium (Calcite, gypsum, anhydrite). Strong glassy has to dim brilliance.
Origin provenance genesis.
Fluorite has a hydatogenesis. Appears in vein, lode, mines, mainly with the sulfides of lead, zinc and silver. Rarer can have pegmatitic genesis. As accessory mineral is in emptinesses of magmatic rocks. Can have a sedimentary origin also, deposited from water in the reserved pools.





Deposit minefield mine field occurrence subsoil.
Found out the best crystals in the English deposits (Uierdeyl near Darkhema, Olston Moore and Clitoris Moore in Kamberlende). It is blocks of green or violet color, in an association with Calcite, barytes, quartz, sphalerite, galena, galenite and siderite. In Canada excellent copies are found in a district near-by Tander Beat, mainly in an association with Amethyst. Other deposits of North America are located in Ontario and Illinois, where the largest industrial developments are. In Europe, besides the English deposits, noteworthy copies from Saxony, having the special yellow-brown tint. Unique on the red color and characteristic octahedral look swiss Fluorites, so-called альпино, obtained mainly in the area of Gottarda. In Italy interesting standards of Fluorite, surprisings on the transparency and colourlessness, are in Korvaro, Альто-Адидже, and in Sarrabuse, on Sardinia. Shpatovidnyy Fluorite widely widespread in the vein, lode, mines of Санта-Барбары at Kollio and Val'-ganna, province of Varese, where it is accompanied galena, galenite. Magnificent violet crystals a-plenty meet in Kamissione near Zogno (Val'-brembana). Shallow, but enough elegant violet crystals are found in a granit cave in Baveno. Rose octahedral crystals of type of " alpino", look like swiss Fluorites from Gottarda, it is possible to find in geode in gneiss Beura and Villadosola (Verbaniya).
Use, practical application, deployment.
Fluorite is the most essential mineral raw material for the receipt preparation of hydrofluoric (hydrofluoric etching) acid. It is also irreplaceable in the production of ceramics and plastics, in optical industry; the most transparent crystals are used for making of lenses and prisms in a spectrography. Finally, Fluorite is used at the meltback of bauxit and as a gumboil is in metallurgy. Varieties. Among the varieties of Fluorite it is needed to mention antozite, dark dark blue-purple, violet, almost black colouring, which the strong smell of free fluorine and ozone is characteristic for, and also хлорофан, green color.

Fluorite. Morocco (north-western part of Africa). Deposit of minefield mine field occurrence subsoil minerals. A photo: © A.A. Evseev

Fluorite (pink, rose), Quartz, Hedenbergite, Vnutr. Mongolia, China. More than 10 sm. A photo: © A.A. Evseev
In the organism of people a fluorine stimulates immune defence and blood formation, hematopoiesis, hematogenesis, promotes stability of teeth to the caries, participates in growth of skeleton, an osteoporosis warns. The table of contents of fluorine in the body of human people makes 2,6 A fluorine at introduction to the organism is very quickly sucked in in an intestine (like chlorides). In an organism a fluorine is in the CPLD state, as difficult-soluble salts with a calcium (Fluorite), magnesium, iron.
A fluorine in an organism stimulates the blood-making hematopoietic system and immunity, stimulates reparative, restorative processes at the breaks of bones, and also warns development of senile osteoporosis. Signs of insufficiency of fluorine: decay of teeth, osteoporosis. Food sources of fluorine: plenty of fluorine is contained in fish (in hardheads, cod and sheat-fish - to 5-10 mcg/g), mutton, veal, in a hepar, nuts (walnut). A lot of fluorine is contained in eggs, milk, spinach. Tea is rich in a fluorine, maintenance of fluorine in him - 75-100 мг% (at brewing to solution 2/3 passes from a general amount). A drinking-water contains a 1 mg of fluorine on a litre.
Suction of fluorine in a gastroenteric highway depends on solubility of their salts and concentration of calcium (Fluorite forms). A fluorine is oppressed by metabolism of iodine and can induce, stimulate a goitre. Magnesium is braked by mastering of fluorine an organism. Some authors cite data that a fluorine also represses acidifier bacteria. A fluorine for a people is an extraordinarily important microelement, as it is vitally needed for normal growth and development.
Displays of surplus of fluorine: appearance of chalk-like spots on teeth ("false teeth", "teeth of shark"), destruction of dental enamel, fragility of teeth, an osteosclerosis (fluorosis) osteomalacia, osteoporosis, calcinosis of vein, lode, mines and copulas, formation of bone spurs; hemorrhage in area of gums, mucous membranes of mouth and nose, loss of voice, dry suffocating cough; bradycardia, brachycardia, bradyrhythmia, decline of arteriotony; itch, irritation and decorticating of epidermis, violations of fatty and carbohydrate exchange. A fluorine is promoted by the risk of origin of osteoblastic (osteogenic, osteoid, osteolytic) sarcoma, osteosarcoma.
DOPOG #2.1 

Flammable gases
Risk of fire. Risk of explosion. It can be under constraint. Risk of difficulty in breathing. Can cause burns and/or frostbite, congelation. Capacities can burst at heating (dangerous, perilous - does not burn practically)
To use shelter. To avoid the low areas of surface (pits, low-laying areas, trenches)
Red rhombus, number of DOPOG, black or white flame
DOPOG #2.3 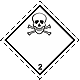
Toxic poisonous gases. Skull and crossed cross-bones
Toxic hazard. It can be under constraint. Can cause burns and/or frostbite, congelation. Capacities can burst at heating (dangerous, perilous is instantaneous distribution of gases on a vinicity)
To use a mask for emergency abandonment of transport vehicle. To use shelter. To avoid the low areas of surface (pits, low-laying areas, trenches)
White rhombus, number of DOPOG, black skull and crossed cross-bones
DOPOG #3 

Flammable liquids
Risk of fire. Risk of explosion. Capacities can burst at heating (dangerous, perilous - burn easily)
To use shelter. To avoid the low areas of surface (pits, low-laying areas, trenches)
Red rhombus, number of DOPOG, black or white flame
DOPOG #6.1 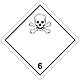
Toxic non biological matters substances (toxin)
Risk of poisoning at inhalation, contact with a derma or swallowing. Make a danger for a water environment or sewage system
To use a mask for emergency abandonment of transport vehicle
White rhombus, number of DOPOG, black skull and crossed cross-bones
